Collective motion in cell sheets
Cells move collectively during the development of an embryo, wound healing or cancer metastases. The coordinated movement of cells depends both on the individual motility of single cells as well as the coupling between neighboring cells and how they interact with the external matrix. Our goal is to understand how cytoskeletal forces are coordinated between cells and their surroundings to produce large scale coherent movements.
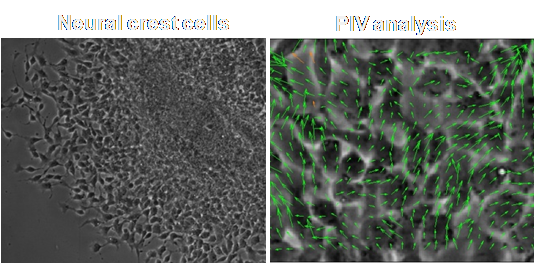
During development, neural crest cells emerge from the embryonic neural tube through a phenomenon called epithelial mesenchymal transition (EMT), migrate to different locations and differentiate into a variety of cell types. As the coordinated of movements of these cells involves extensive physical interactions with the surroundings, the mechanical properties of the environment may be an important regulator of movement. In collaboration with Dr. Lisa Taneyhill, we use a 2D monolayer system to quantify the movement of cells as they migrate out as a monolayer. Our initial results show novel dynamics of single cells and groups of cells indicative of significant stresses in the system.
Lab Members Involved: Shen Li, Christy Ketchum
Collaborators: Taneyhill Lab, UMD Animal and Avian Sciences
Funding: UMD Advance Grant

Mechanics of in vitro and cellular actin networks
Various actin-binding proteins crosslink actin filaments and organize
them into diverse structures. Palladin is a newly identified actin
cross-linking protein that is important in organizing cellular actin
networks. Genetic ablation of palladin is lethal and mutations in
palladin impair embryonic development and cell movement. These deficits
may arise because the cellâs ability to adjust its internal stiffness is
compromised in the absence of palladin. We are studying how palladin, by
its ability to cross-link actin and its interaction with another actin
cross-linker, alpha-actinin, determines the structure and mechanical
properties of actin networks and enables the cell to sense its physical
environment. These studies will shed light on how actin organization
impacts cellular mechano-sensitivity.
Lab Members Involved: Brian Grooman
Collaborators: Otey
Lab, UNC Chapel Hill
Grant/Fellowship: NSF-MCB
Actin dynamics in B cell signaling
B lymphocytes form a critical part of the immune response by generating antibody responses against invading pathogens and maintaining long lasting immunity. Binding of antigens to clonally specific B cell receptors (BCR) initiates B cell activation and the formation of signaling microclusters of antigen-engaged BCR. Despite its considerable importance, physical insight into the mechanisms of microcluster assembly is limited. Using a combination of TIRF, single molecule imaging and nano-fabrication techniques, we are studying how mesoscale reorganization of BCR is regulated by internal parameters such as the dynamics of the actin cytoskeleton as well as external parameters such as ligand mobility and surface topography.
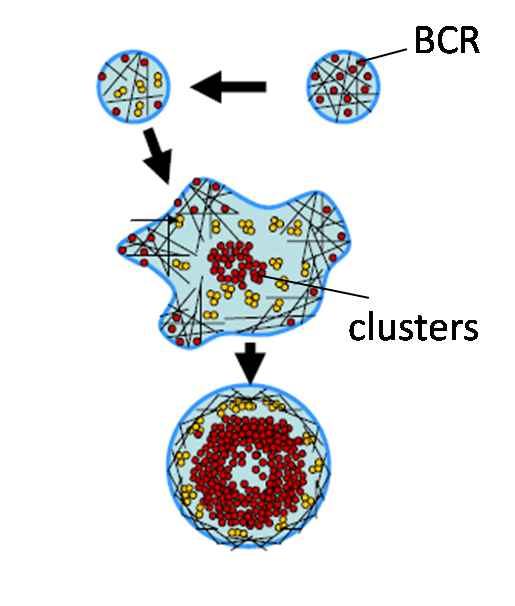
Ligand mobility and BCR signaling: We study the interaction of B cells with stimulatory glass or planar lipid bilayer surfaces to examine the differences in BCR response to immobile and mobile ligands. We have shown that ligand mobility is an important parameter regulating microcluster formation, movement and signaling. Using dual wavelength TIRF to simultaneously visualize actin and BCR movement, we found that actin dynamics can directly drive the movement of receptor clusters [Ketchum et al, 2013].
Actin mediated signaling in B cells: In collaboration with Dr. Wenxia Song, we have also shown that the BCR microclusters actively regulate actin cytoskeleton dynamics. Actin polymerization in turn drives BCR clustering and aggregation, indicating that these cellular components (actin cytoskeleton, receptor clusters and signaling activation) comprise a feedback loop that drives B cell activation [Liu et al., 2011, Liu et al., 2012].
Lab Members Involved: Christy Ketchum
Collaborators: Song
Lab, UMD Cell Biology &
Fourkas Lab, UMD Chemistry
Grant/Fellowship: UMD Advance Grant and ARCS Fellowship
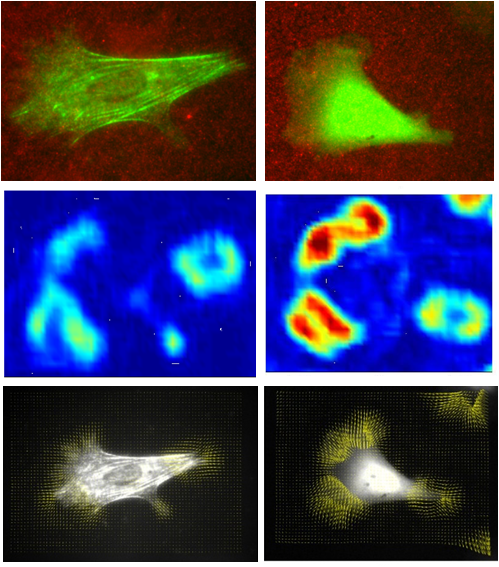
Actin crosslinkers and cellular mechanosensing
The ability of cells to sense the mechanical stiffness of their
environment is critical for many aspects of cell function such as
migration, wound healing and the proper formation of tissues and organs.
In order to sense the mechanical properties of their environment, cells
adjust their internal stiffness to match that of the external surface by
reorganizing their actin cytoskeleton: a network of polymer filaments.
Lab Members Involved: Mikheil Azatov
Collaborators: Otey
Lab, UNC Chapel Hill
Grant/Fellowship: NSF-MCB




