Physical aspects of T cell signaling
The interplay of physical forces and biochemical signaling pathways is critical in driving many biological phenomena including the immune response. T cells, which are central players in the immune response, bind to antigen presenting cells through specialized receptor-ligand interactions. Receptor engagement initiates the reorganization of the actin cytoskeleton which causes deformations of the cell membrane and spatial patterning of signaling proteins into micro-clusters which are the fundamental units of signaling. The physical regulation of this self-organization process, a vital step in the immune response, is not well understood. We aim to understand the physical mechanisms governing the formation of contact between immune cells during signaling activation and spatial organization of signaling proteins.
Membrane fluctuations:
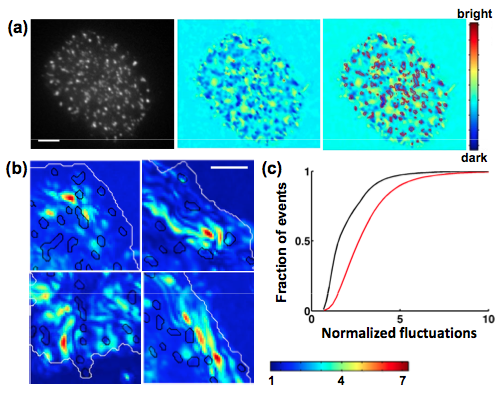 Biochemical signaling at interfaces is subject to physical constraints such as membrane topography and membrane bending which modulate receptor ligand interactions. Fluctuations normal and tangential to the surface affect the lifetime of bonds and interaction energies between receptors and ligands. We used Interference Reflection Microscopy to examine the membrane topography and nanometer scale height fluctuations of Jurkat T cell membranes in contact with an activating surface. We found that the locations of signaling clusters were correlated with domains of strong adhesion and low fluctuations, indicating that membrane topography is an important parameter governing the spatial assembly of signaling [Hui et al. Biophys J, 2011]. Our results highlight the role of active membrane fluctuations in driving surface receptor reorganization - critical for the regulation of immune responses.
Biochemical signaling at interfaces is subject to physical constraints such as membrane topography and membrane bending which modulate receptor ligand interactions. Fluctuations normal and tangential to the surface affect the lifetime of bonds and interaction energies between receptors and ligands. We used Interference Reflection Microscopy to examine the membrane topography and nanometer scale height fluctuations of Jurkat T cell membranes in contact with an activating surface. We found that the locations of signaling clusters were correlated with domains of strong adhesion and low fluctuations, indicating that membrane topography is an important parameter governing the spatial assembly of signaling [Hui et al. Biophys J, 2011]. Our results highlight the role of active membrane fluctuations in driving surface receptor reorganization - critical for the regulation of immune responses.
Actin dynamics in T cells: Actin reorganization is a critical aspect of the T cell immune response and formation of the immune synapse. Using TIRF microscopy, we have visualized the dynamics of actin, actin regulatory proteins and signaling molecules during the early stages Jurkat T cell spreading on a stimulating surface. We found that the actin cytoskeleton organizes into non-equilibrium patterns such as membrane coupled actin waves which appear to emerge from signaling clusters [Hui et al., 2013]. Actin-membrane waves may enable cells to dynamically scan the target surface, increasing receptor-ligand interactions and subsequent T cell signaling.
Cytoskeletal forces and T cell signaling: The link between physical forces and TCR activation is an open question in the field of T cell signaling. Recent studies have shown that externally applied forces can activate TCR signaling, indicating a potential role for forces. We have examined the role of internal cytoskeletal forces during T cell signaling activation. We used traction force microscopy to directly measure the forces generated during Jurkat T cell spreading on a stimulatory surface. We found that T cell forces are largely generated by actin assembly dynamics with limited contribution from myosin. Jurkat T cells also exhibit mechanosensitivity to substrate stiffness, exerting larger forces on stiffer surfaces. The morphology and signaling was also found to be sensitive to the stiffness of the activating substrate. Our results suggest that actin generated forces may be important for signal transduction in T cells.
Lab Members Involved: King Lam Hui
Collaborators: Papoian
Lab, UMD Chemistry
Mechanics of in vitro and cellular actin networks
Actin-binding proteins crosslink actin filaments into viscoelastic gels and organize them into diverse cellular structures such as bundles or meshworks. Small changes in binding energies can cause the system to switch from network dominated to bundle dominated regimes. These crosslinkers determine the length and orientational order of filaments and the inter-filament spacing, and thereby control the response of the network to external forces. Palladin is a newly identified actin crosslinking protein that is important in organizing cellular actin networks and critical for cell movements and morphology. In addition to binding actin palladin also binds other actin associated proteins such as alpha-actin and several signaling intermediaries.

Mechanical properties of actin networks: We have studied how palladin, by its ability to cross-link actin and its interaction with another actin cross-linker, alpha-actinin, determines the structure and mechanical properties of actin networks. Using a reconstituted system of composite actin networks crosslinked with palladin and alpha-actinin, we have found that these mutually interacting crosslinkers synergistically modulate network viscoelastic properties [Grooman et al., PLoS One, 2012].

Force transmission in cellular actin bundles: Cells encounter diverse forces as they move through extracellular matrix or tissues. To study how forces are transmitted through cells and groups of cells, we use magnetic tweezers to directly apply external forces to cells and measure their response by quantifying the strain changes in actin bundles called stress fibers. Local application of force leads to a global cellular response, which depends on various physical parameters of the applied force.
Lab Members Involved: Brian Grooman, Shirong Zhang
Collaborators: Otey
Lab, UNC Chapel Hill
Grant/Fellowship: NSF-MCB
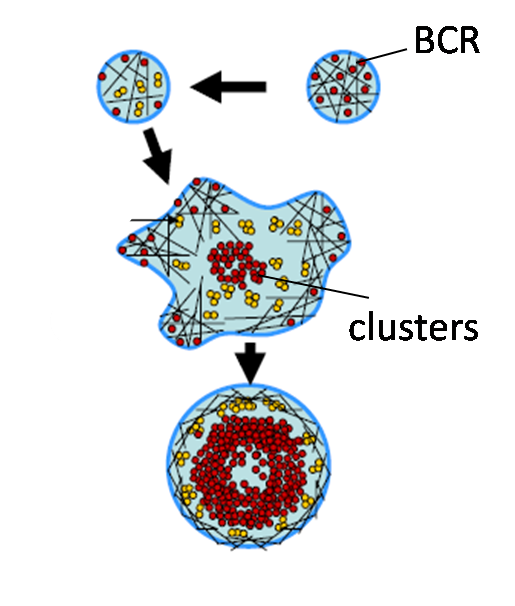
Actin dynamics in B cell signaling
B lymphocytes comprise a critical part of the immune response by generating antibody
responses against invading pathogens and maintaining long lasting immunity in the body.
Binding of antigens (Ag) to clonally specific B cell receptors (BCR) initiates B cell activation, ultimately leading to antibody production.
Antigen binding induces receptor self-aggregation, formation of signaling microclusters, cell spreading
and signaling activation - a series of complex spatiotemporal events. The dynamics and
organization of surface BCRs is critical for receptor activation. While the biochemical
signaling and regulatory pathways governing early B cell signaling have been well studied,
the role of the physical properties of antigen binding has not been fully examined. Using a combination of
total internal reflection microscopy, single molecule imaging and nano-fabrication techniques, we are studying how mesoscale reorganization of BCR is regulated by internal parameters such as the dynamics of the actin cytoskeleton as well as external parameters such as ligand mobility and surface topography.
Lab Members Involved: Christy Ketchum
Collaborators: Song
Lab, UMD Cell Biology &
Fourkas Lab, UMD Chemistry
Grant/Fellowship: UMD Advance Grant and ARCS Fellowship
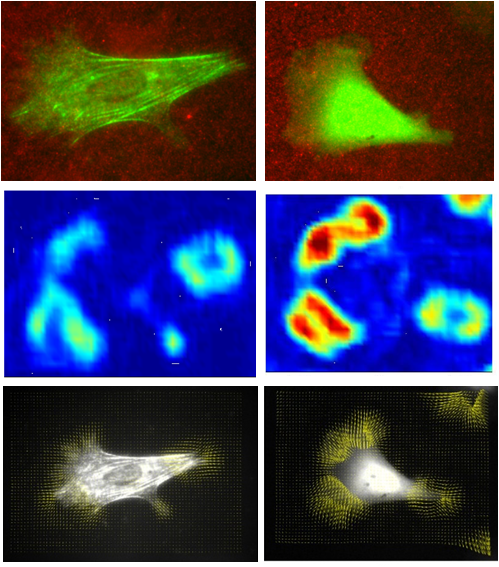
Actin crosslinkers and cellular mechanosensing
The ability of cells to sense the mechanical stiffness of their
environment is critical for many aspects of cell function such as
migration, wound healing and the proper formation of tissues and organs.
In order to sense the mechanical properties of their environment, cells
adjust their internal stiffness to match that of the external surface by
reorganizing their actin cytoskeleton: a network of polymer filaments.
Lab Members Involved: Mikheil Azatov
Collaborators: Otey
Lab, UNC Chapel Hill
Grant/Fellowship: NSF-MCB





